Identify the mole ratios in the following equations:
1. CaBra2 + Na= NaBr + Ca
2. 2H20 (I)=H...

Chemistry, 06.05.2021 21:00 wolfgirl4762
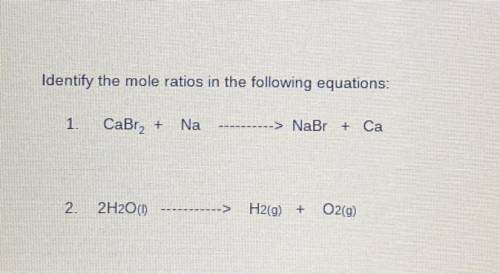
Identify the mole ratios in the following equations:
1. CaBra2 + Na= NaBr + Ca
2. 2H20 (I)=H2(g) + O2(9)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Questions



Physics, 20.12.2020 03:10




Mathematics, 20.12.2020 03:10

Health, 20.12.2020 03:10

Mathematics, 20.12.2020 03:10


History, 20.12.2020 03:10

Social Studies, 20.12.2020 03:10



Health, 20.12.2020 03:10

Mathematics, 20.12.2020 03:10


Mathematics, 20.12.2020 03:10


Health, 20.12.2020 03:10



