Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 11:40
Modern pennies are composed of zinc coated with copper. a student determines the mass of a penny to be 2.482 g and then makes several scratches in the copper coaling (to expose the underlying zinc). the student puts the scratched penny in hydrochloric acid, where the following reaction occurs between the zinc and the hcl (the copper remains undissolved): zn(s) + 2 hcl(aq) → h2(g) + zncl(aq)the student collects the hydrogen produced over water at 25 °c. the collected gas occupies a volume of 0.899 l at a total pressure of 79 j mmhg. calculate the percent zinc (by mass) in the penny. (assume that all the zn in the penny dissolves.)
Answers: 1

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

You know the right answer?
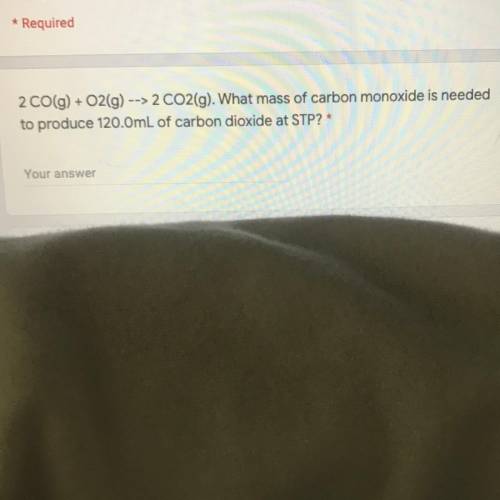
What mass of carbon monoxide is needed to produce 120.0mL of carbon dioxide at STP?
(show work ple...
Questions



Mathematics, 01.04.2021 18:40


Mathematics, 01.04.2021 18:40










Health, 01.04.2021 18:40


Mathematics, 01.04.2021 18:40

Mathematics, 01.04.2021 18:40

Biology, 01.04.2021 18:40

Mathematics, 01.04.2021 18:40