
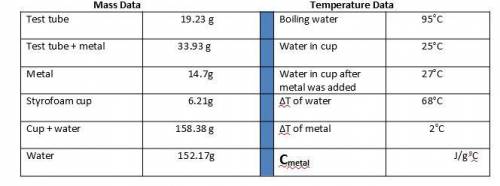
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation in the introduction.
Metal A
Q = mc(ΔT)
Qwater = -Qmetal
Heat gained = Mass of x Specific heat of x Change in temperature
by the water water (g) water (4.184 J/goC) (ΔT)
The specific heat of the metal can now be calculated:
Specific heat = Heat gained by the water
of metal (c) Mass of metal (g) x ΔT of metal (oC)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation...
Questions

Mathematics, 01.06.2021 04:40

Biology, 01.06.2021 04:40



Mathematics, 01.06.2021 04:40


Mathematics, 01.06.2021 04:50

Mathematics, 01.06.2021 04:50

Geography, 01.06.2021 04:50


Mathematics, 01.06.2021 04:50



English, 01.06.2021 04:50


Mathematics, 01.06.2021 04:50


Social Studies, 01.06.2021 04:50


Mathematics, 01.06.2021 04:50




