
Chemistry, 07.05.2021 03:20 Honeyswish7730
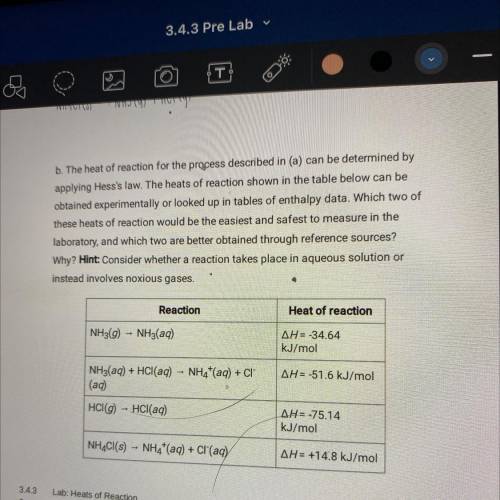
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law. The heats of reaction shown in the table below can be
obtained experimentally or looked up in tables of enthalpy data. Which two of
these heats of reaction would be the easiest and safest to measure in the
laboratory, and which two are better obtained through reference sources?
Why? Hint: Consider whether a reaction takes place in aqueous solution or
instead involves noxious gases.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 00:30
Sarah wants to know where in her garden chamomile would grow the best. she thinks chamomile will grow best in the corner of the garden that gets the most sunlight. to test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. which of the following variables will sarah need to measure to know which group of plants grew best? a. the location of the plants b. the type of plants c. the height of the plants d. the amount of water she gives the plants
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law....
Questions

Computers and Technology, 17.09.2019 05:20






Mathematics, 17.09.2019 05:20


Mathematics, 17.09.2019 05:20

English, 17.09.2019 05:20






Mathematics, 17.09.2019 05:20

Mathematics, 17.09.2019 05:20

English, 17.09.2019 05:20


Mathematics, 17.09.2019 05:20



