
1. Calculation of equilibrium concentrations from Ka
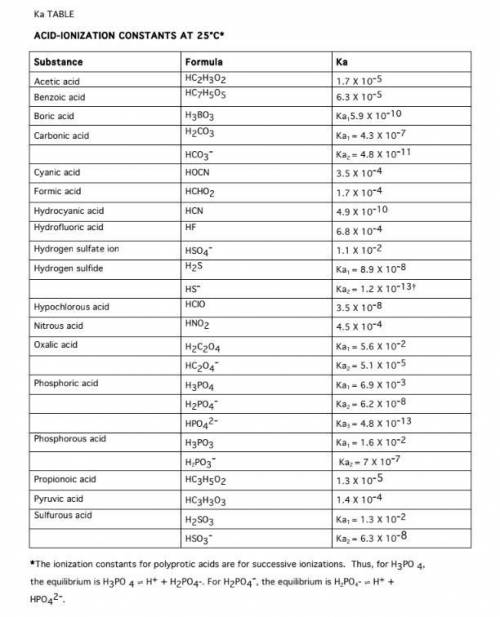
Calculate the pH of a 1.0 M Acetic acid solution, using approximations. Ka = 1.8 x 10^-5
HC2H3O2+H2O⇆H3O(+)+C2H3O2(-)
Initial:
Change
at Equilibrium:
2. Calculation of species concentrations from Ka, using the quadratic formula
Calculate the pH of a 0.000010 M Acetic acid solution
HC2H3O2+H2O⇆H3O(+)+C2H3O2(-)
Initial:
Change:
at Equilibrium:
3. Calculation of Ka from the pH of a weak acid solution
Calculate the Ka of HNO2 if a 0.10 M HNO2 solution has a pH of 2.187
Initial:
Change:
at Equilibrium:
4. Calculation of Ka from the percent ionization
Calculate the Ka of Glycine if a 0.10 M Glycine solution is 4.1 x 10^-3 ionized
HGly+H2O⇆H3O(+)+Gly(-)
Initial:
Change:
at Equilibrium:

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
1. Calculation of equilibrium concentrations from Ka
Calculate the pH of a 1.0 M Acetic acid solut...
Questions




Mathematics, 07.01.2020 21:31

English, 07.01.2020 21:31

Computers and Technology, 07.01.2020 21:31

History, 07.01.2020 21:31



History, 07.01.2020 21:31

Mathematics, 07.01.2020 21:31

Spanish, 07.01.2020 21:31

Chemistry, 07.01.2020 21:31

English, 07.01.2020 21:31

Mathematics, 07.01.2020 21:31

Mathematics, 07.01.2020 21:31


Chemistry, 07.01.2020 21:31

Physics, 07.01.2020 21:31

English, 07.01.2020 21:31




