Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
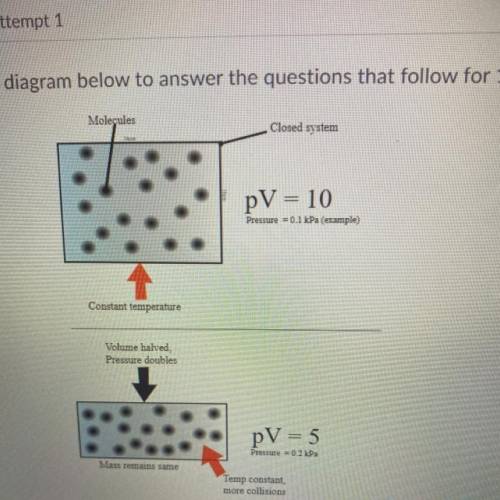
Use the diagram below to answer the question that follow for 1 point each
1. identify what conditi...
Questions


English, 04.06.2020 23:05

Mathematics, 04.06.2020 23:05


Biology, 04.06.2020 23:05



History, 04.06.2020 23:05

Law, 04.06.2020 23:05

Mathematics, 04.06.2020 23:05



Mathematics, 04.06.2020 23:05

Chemistry, 04.06.2020 23:05

Health, 04.06.2020 23:05

Health, 04.06.2020 23:05




Chemistry, 04.06.2020 23:05