Someone please help!!
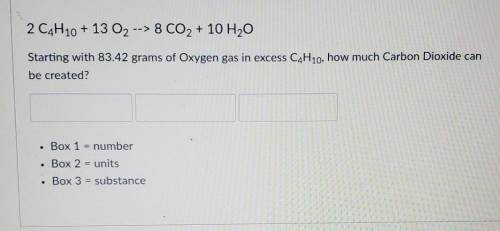
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxyg...

Chemistry, 07.05.2021 22:00 xMABRYx1991
Someone please help!!
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxygen gas in excess C4H10, how much Carbon Dioxide can be created?
Box 1 = number
Box 2 = units
Box 3 = substance

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1
You know the right answer?
Questions

English, 10.03.2021 21:00


Mathematics, 10.03.2021 21:00


Mathematics, 10.03.2021 21:00

Chemistry, 10.03.2021 21:00






English, 10.03.2021 21:00


History, 10.03.2021 21:00

Mathematics, 10.03.2021 21:00

Physics, 10.03.2021 21:00

History, 10.03.2021 21:00


English, 10.03.2021 21:00

Chemistry, 10.03.2021 21:00




