
Chemistry, 07.05.2021 22:20 keananashville
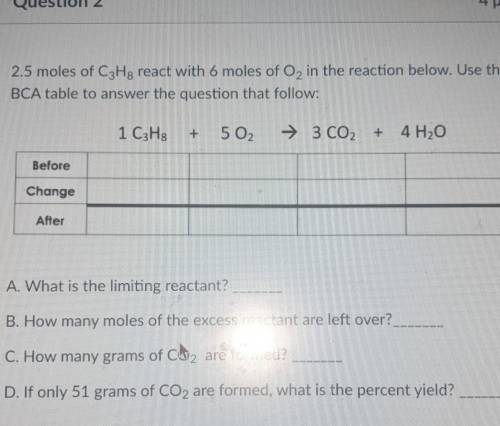
2.5 moles of C3H8 react with 6 moles of O2 in the reaction below. Use the BCA table to answer the question that follow: 1 C3H8 + 5 02 → 3 CO2 + 4H2O
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?.
C. How many grams of CO2 are formed?
D. If only 51 grams of CO2 are formed, what is the percent yield?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
2.5 moles of C3H8 react with 6 moles of O2 in the reaction below. Use the BCA table to answer the qu...
Questions

History, 05.11.2019 08:31





Mathematics, 05.11.2019 08:31


Mathematics, 05.11.2019 08:31

Social Studies, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31


Mathematics, 05.11.2019 08:31



Mathematics, 05.11.2019 08:31



Social Studies, 05.11.2019 08:31

Mathematics, 05.11.2019 08:31



