
Chemistry, 07.05.2021 22:30 aallyssabrown0120
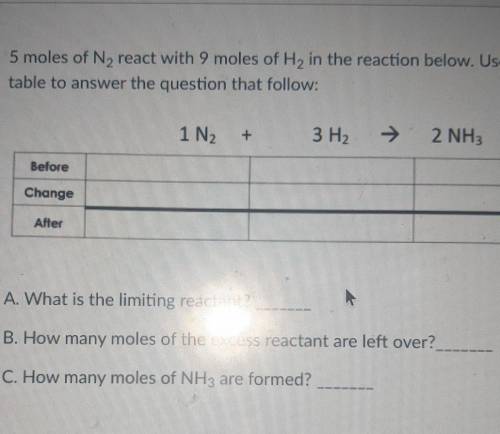
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the question that follow: 1N2+3H2=2NH3
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?
C. How many moles of NH3 are formed?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the questi...
Questions

SAT, 21.05.2021 20:10

Mathematics, 21.05.2021 20:10

Health, 21.05.2021 20:10


Advanced Placement (AP), 21.05.2021 20:10

Mathematics, 21.05.2021 20:10




Chemistry, 21.05.2021 20:10

English, 21.05.2021 20:10

Mathematics, 21.05.2021 20:10

Social Studies, 21.05.2021 20:10

History, 21.05.2021 20:10


Mathematics, 21.05.2021 20:10



Mathematics, 21.05.2021 20:10




