
Chemistry, 08.05.2021 01:00 mckenziealexander
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaOH (aq) + H2 (g). If a teacher uses a 0.500 gram sample of sodium metal for a demonstration with excess water, how many liters of hydrogen gas would be produced at STP?
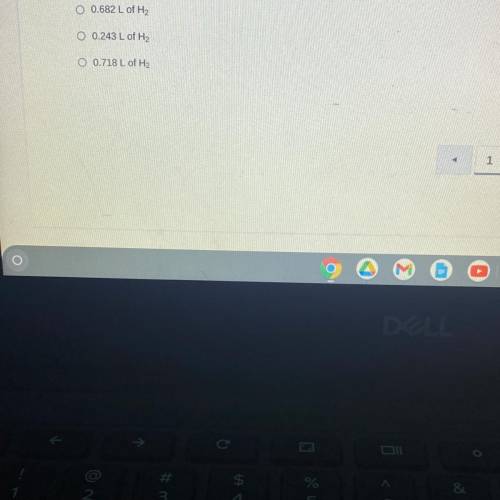

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaO...
Questions


Mathematics, 29.05.2021 06:20

Mathematics, 29.05.2021 06:20

Chemistry, 29.05.2021 06:20

Mathematics, 29.05.2021 06:20

Mathematics, 29.05.2021 06:20



History, 29.05.2021 06:30

Mathematics, 29.05.2021 06:30

Mathematics, 29.05.2021 06:30




Health, 29.05.2021 06:30

Mathematics, 29.05.2021 06:30

Mathematics, 29.05.2021 06:30





