
Chemistry, 08.05.2021 02:00 zchwilke2981
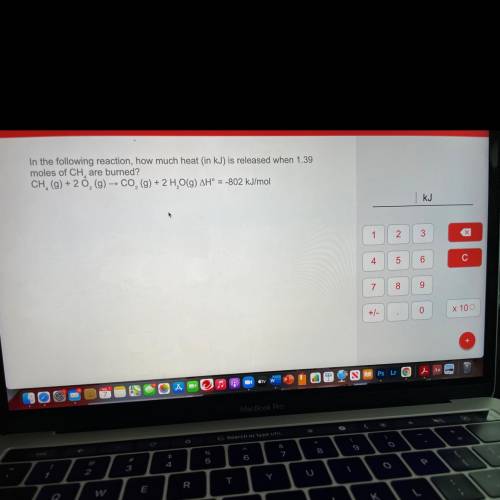
In the following reaction, how much heat (in kJ) is released when 1.39
moles of CH are burned?
CH, (g) + 20, (g) → CO2 (g) + 2 H, O(9) AH° = -802 kJ/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 11:30
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3

Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
In the following reaction, how much heat (in kJ) is released when 1.39
moles of CH are burned?
Questions


Spanish, 04.04.2020 07:35



History, 04.04.2020 07:35


Mathematics, 04.04.2020 07:35



SAT, 04.04.2020 07:35


English, 04.04.2020 07:35





Computers and Technology, 04.04.2020 07:36





