Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
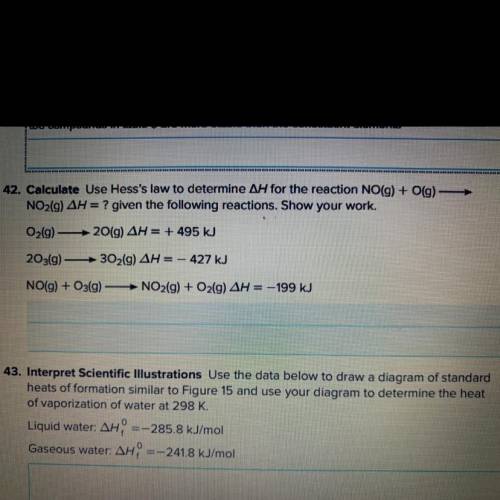
Calculate, use hess’s law to determine /\H for the reaction NO(g) + O(g)—> NO2(g) /\H=? given the...
Questions


Computers and Technology, 03.01.2021 06:10

Arts, 03.01.2021 06:10


Mathematics, 03.01.2021 06:10

Advanced Placement (AP), 03.01.2021 06:10

English, 03.01.2021 06:10

Computers and Technology, 03.01.2021 06:10



Mathematics, 03.01.2021 06:10






Health, 03.01.2021 06:10


Biology, 03.01.2021 06:10