
Chemistry, 08.05.2021 08:10 sparky1234
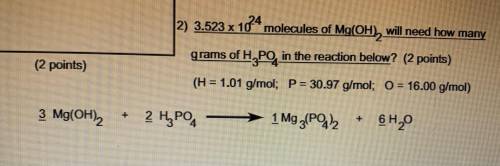
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01 g/mol
P=30.97 g/mol
O=16.00 g/mol
3 Mg(OH)2 + 2 H3PO4 -> 1 Mg3 (PO4 )2 + 6 H2O

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
3.523 x 10^24 molecules of Mg(OH2) will need how many grams of H3PO4 in the reaction below
H=1.01...
Questions




Mathematics, 11.11.2020 19:50


Geography, 11.11.2020 19:50

Mathematics, 11.11.2020 19:50

Chemistry, 11.11.2020 19:50

Spanish, 11.11.2020 19:50

Chemistry, 11.11.2020 19:50


Mathematics, 11.11.2020 19:50










