
Chemistry, 08.05.2021 22:50 lerasteidl
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium carbonate when 2 moles of HCl are used to start with?
2HCl + CaCO3 +CaCl2 + CO2 + H2O
A. 2
B. 4
C. 1
D. 3
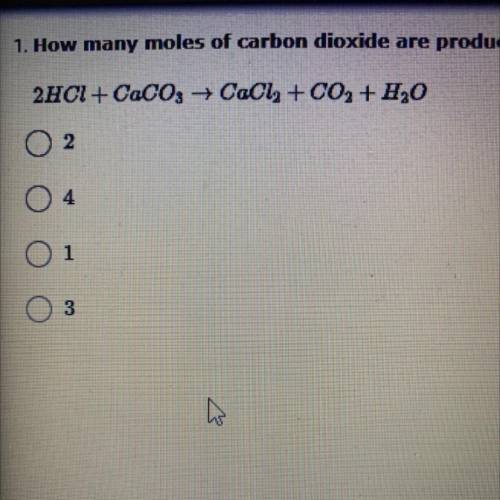

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
How many moles of carbon dioxide are produced in the reaction between hydrochloric acid and calcium...
Questions





Mathematics, 21.10.2020 01:01




Medicine, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01



History, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01

Computers and Technology, 21.10.2020 01:01


Mathematics, 21.10.2020 01:01

Mathematics, 21.10.2020 01:01

History, 21.10.2020 01:01



