
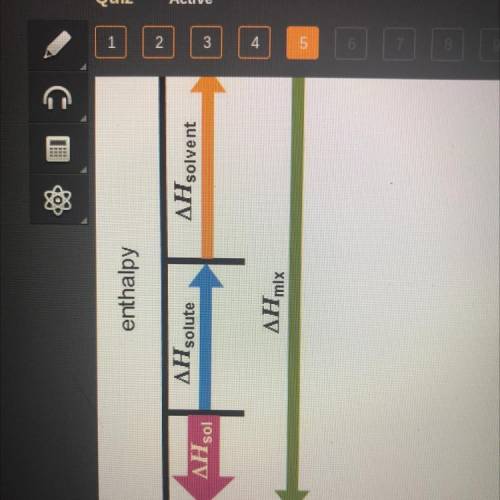
Which statement accurately describes the diagram?
The temperature increases after dissolution, and the process iS exothermic.
The temperature increases after dissolution, and the process is endothermic.
The temperature decreases after dissolution, and the process
is exothermic.
The temperature decreases after dissolution, and the process is endothermic.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Which statement accurately describes the diagram?
The temperature increases after dissolution, and...
Questions





Mathematics, 11.03.2020 02:09






Mathematics, 11.03.2020 02:09

English, 11.03.2020 02:09


English, 11.03.2020 02:09




Mathematics, 11.03.2020 02:09


Geography, 11.03.2020 02:09



