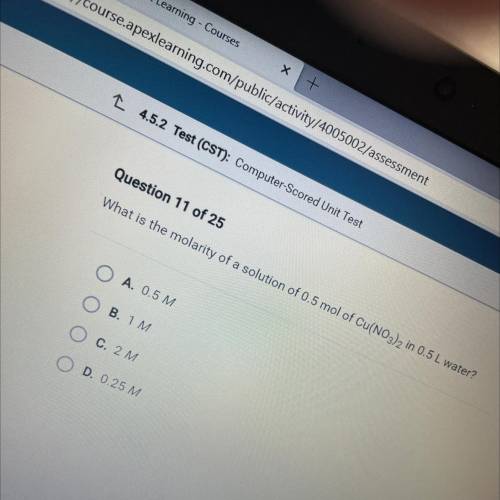
What is the molarity of a solution of 0.5 mol of Cu(NO3)2 in 0.5 L water?
...

Chemistry, 10.05.2021 01:40 lucystudies
What is the molarity of a solution of 0.5 mol of Cu(NO3)2 in 0.5 L water?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
Questions

History, 10.03.2021 17:40


Mathematics, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40


Social Studies, 10.03.2021 17:40

Spanish, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40

Chemistry, 10.03.2021 17:40

History, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40

Computers and Technology, 10.03.2021 17:40

Chemistry, 10.03.2021 17:40



Mathematics, 10.03.2021 17:40

Mathematics, 10.03.2021 17:40


Mathematics, 10.03.2021 17:40



