
Chemistry, 10.05.2021 14:00 amiechap12
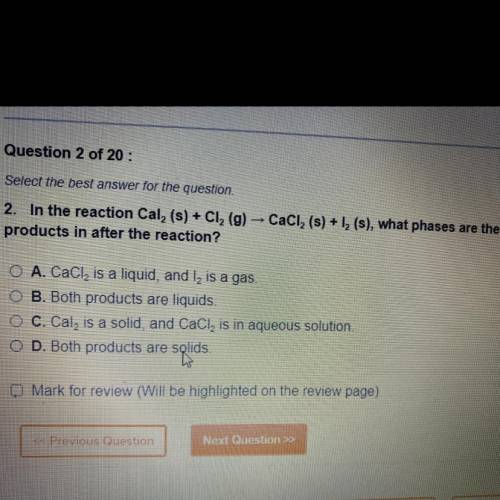
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the reaction ?
A. CaCl 2 is a liquid, and l2 is a gas
B. Both products are a liquid
C. Cal2 is a solid, CaCl2 is in aqueous solution
D. Both products are solids

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

You know the right answer?
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the re...
Questions

Mathematics, 04.10.2021 08:30

Advanced Placement (AP), 04.10.2021 08:30

English, 04.10.2021 08:30

Mathematics, 04.10.2021 08:30


Mathematics, 04.10.2021 08:30


Mathematics, 04.10.2021 08:30


History, 04.10.2021 08:30

History, 04.10.2021 08:30

Mathematics, 04.10.2021 08:30






Mathematics, 04.10.2021 08:30

Business, 04.10.2021 08:30

Physics, 04.10.2021 08:30



