Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
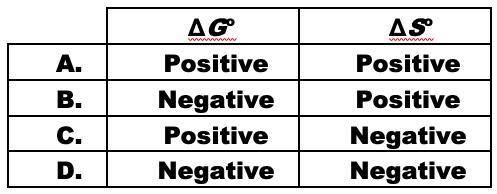
When ammonium nitrate dissolves in water, the solution becomes cold.
NH4NO3(s) <-> NH4+(aq)...
Questions






Mathematics, 20.10.2019 11:20


History, 20.10.2019 11:20



English, 20.10.2019 11:20

History, 20.10.2019 11:20


Biology, 20.10.2019 11:20

Chemistry, 20.10.2019 11:20




Social Studies, 20.10.2019 11:20

History, 20.10.2019 11:20