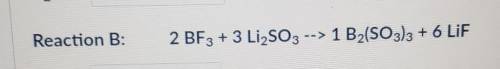Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
According to Reaction B, how many moles of B2(SO3)3 can be formed from 9.31 moles of LiF?
3.10 mol...
Questions

Mathematics, 12.05.2021 23:40


English, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40



Social Studies, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40

Physics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40


Mathematics, 12.05.2021 23:40

Mathematics, 12.05.2021 23:40