
Chemistry, 11.05.2021 08:00 87haymaker
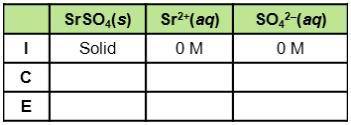
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E) stand for in the table? How can the table be used to find equilibrium constants for this example?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1
You know the right answer?
Consider the dissociation of SrSO4, which has a Ksp of 3.2 x 10-7. What do the three rows of (I, C,E...
Questions

Mathematics, 31.01.2020 13:49





Mathematics, 31.01.2020 13:49


Mathematics, 31.01.2020 13:49


World Languages, 31.01.2020 13:49

History, 31.01.2020 13:49

Mathematics, 31.01.2020 13:49


Chemistry, 31.01.2020 13:49

Biology, 31.01.2020 13:49







