
Chemistry, 11.05.2021 18:50 1r32tgy5hk7
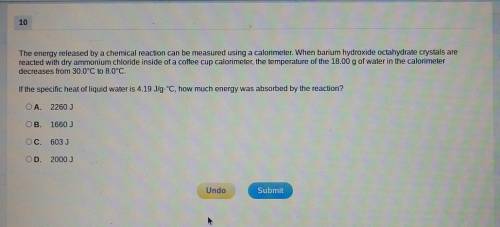
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxide octahydrate crystals are reacted with dry ammonium chloride inside of a coffee cup calorimeter, the temperature of the 18.00g of water in the calorimeter decreases from 30.0⁰C to 8.0⁰C. If the specific heat of liquid water is 4.19J/g.⁰C, how much energy was absorbed by the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
The energy released by a chemical reaction can be measured using a calorimeter. When barium hydroxid...
Questions


English, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

English, 28.07.2020 21:01

English, 28.07.2020 21:01




Arts, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01



Mathematics, 28.07.2020 21:01

Mathematics, 28.07.2020 21:01

Social Studies, 28.07.2020 21:01


Mathematics, 28.07.2020 21:01



