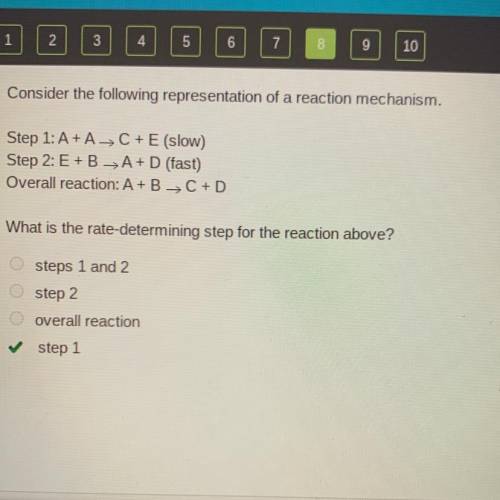
Consider the following representation of a reaction mechanism.
Step 1: A+ A-> C+E (slow)
S...

Chemistry, 11.05.2021 19:40 powella033
Consider the following representation of a reaction mechanism.
Step 1: A+ A-> C+E (slow)
Step 2: E+BA+D (fast)
Overall reaction: A+B C + D
What is the rate-determining step for the reaction above?
steps 1 and 2
step 2
overall reaction
step 1
To help others in the future

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Jewelweed, a flowering plant, has seed pods that burst open when touched and forcefully eject their seeds. these structures are favorable because they a. can cause genetic changes to occur. b. prevent germination within the seed pod. c. aid in the dispersal of the species. d. attract insects that aid in pollination.
Answers: 3


Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
You know the right answer?
Questions



Social Studies, 16.01.2021 03:30

Biology, 16.01.2021 03:30


Social Studies, 16.01.2021 03:30

English, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

Chemistry, 16.01.2021 03:30

Law, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

Mathematics, 16.01.2021 03:30

History, 16.01.2021 03:30


Mathematics, 16.01.2021 03:30





