
Chemistry, 11.05.2021 21:20 fhbuvgy7836
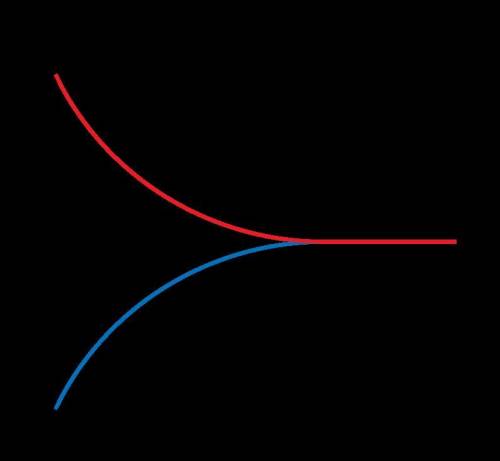
What is true in this reaction?
The reaction will go to completion because the rate of the forward reaction is greater than the rate of the reverse reaction.
The reaction does not reach equilibrium because the rates of the forward and reverse reactions are different.
The reaction reaches chemical equilibrium when the rates of the forward and reverse reactions are equal.
Whether the reaction is at equilibrium cannot be determined by looking at the graph.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1
You know the right answer?
What is true in this reaction?
The reaction will go to completion because the rate of the forward r...
Questions




English, 10.03.2020 17:02


Mathematics, 10.03.2020 17:02

Mathematics, 10.03.2020 17:02




Health, 10.03.2020 17:02






History, 10.03.2020 17:02



Computers and Technology, 10.03.2020 17:02



