
Chemistry, 12.05.2021 14:00 joelpimentel
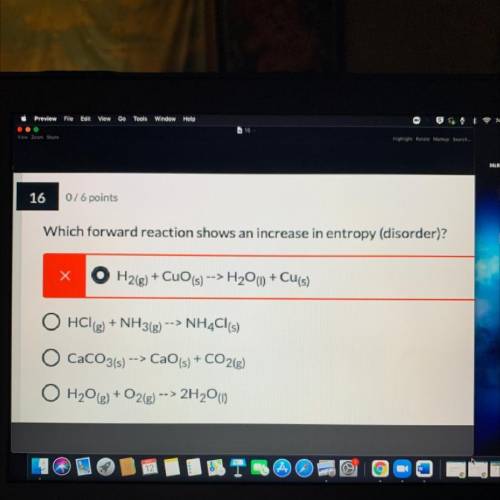
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2O(l) + Cu(s)
B. HCl(g) + NH3(g) --> NH4Cl(s)
C. CaCO3(s) -> CaO(s) + CO2g)
D. H2O(g) + O2(g) --> 2H2O)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2...
Questions

English, 21.09.2019 16:30




Mathematics, 21.09.2019 16:30


Mathematics, 21.09.2019 16:30




History, 21.09.2019 16:30

Chemistry, 21.09.2019 16:30



Mathematics, 21.09.2019 16:30

Mathematics, 21.09.2019 16:30

Mathematics, 21.09.2019 16:30






