
Chemistry, 12.05.2021 16:00 yasminothman02
When the equation below is balanced using the smallest whole number coefficients, what is the sum of all the coefficients?
C2H4(g) + LO2(g) → CO2(g) +
H₂O1
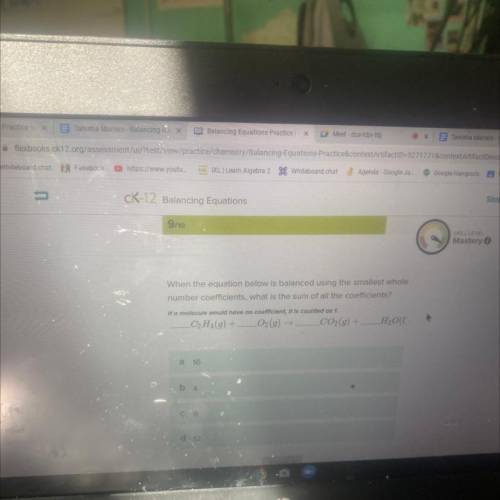

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 02:30
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
When the equation below is balanced using the smallest whole number coefficients, what is the sum of...
Questions


Business, 01.12.2020 19:00

Health, 01.12.2020 19:00

History, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00

Chemistry, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00



Mathematics, 01.12.2020 19:00

Health, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00

Arts, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00

Health, 01.12.2020 19:00

Mathematics, 01.12.2020 19:00


Mathematics, 01.12.2020 19:00



