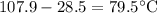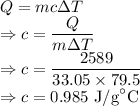Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
A 33.05 g sample of a substance is initially at 28.5 °C. After absorbing 2589 J of heat, the tempera...
Questions

History, 06.10.2020 01:01


Biology, 06.10.2020 01:01



History, 06.10.2020 01:01


Physics, 06.10.2020 01:01

Mathematics, 06.10.2020 01:01



Mathematics, 06.10.2020 01:01




Mathematics, 06.10.2020 01:01

Mathematics, 06.10.2020 01:01


History, 06.10.2020 01:01

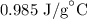
 = Change in temperature =
= Change in temperature =