
Chemistry, 13.05.2021 03:40 jesussaves333
Which of the following reactions is exothermic? A. light + 6CO2 + 6H2O → C8H12O6 + 6O2 B. 2HgO (s) + heat → 2Hg(I) + O2(g) C. Mercury oxide plus heat yields mercury and oxygen D. None of the reactions are exothermic.
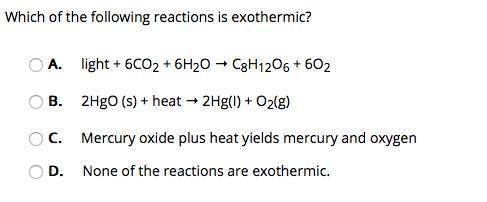

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


You know the right answer?
Which of the following reactions is exothermic? A. light + 6CO2 + 6H2O → C8H12O6 + 6O2 B. 2HgO (s) +...
Questions






















