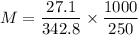Chemistry, 13.05.2021 05:30 PastelHibiscus
A solution was prepared by dissolving 27.1g of sucrose (C12H22O11) in 250 g of water. Find the molality of the solution(molar mass of C12H22O11 is 342.8)
-.108 m
-108 m
-317 m
-.317 m

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
A solution was prepared by dissolving 27.1g of sucrose (C12H22O11) in 250 g of water. Find the molal...
Questions

History, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23





Chemistry, 20.04.2020 05:23

English, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23

Biology, 20.04.2020 05:23

Mathematics, 20.04.2020 05:23





History, 20.04.2020 05:23

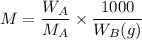
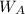 = mass of the solute = 27.1 g
= mass of the solute = 27.1 g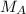 = molar mass of the solute = 342.8
= molar mass of the solute = 342.8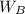 = mass of the given solvent = 250 g
= mass of the given solvent = 250 g