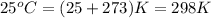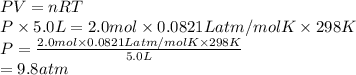Chemistry, 13.05.2021 09:40 laylac45531
A sample of 2.0 moles of helium gas is contained in a tank with a volume of 5.0L at a temperature of 25°C. What is the pressure of the gas in the tank
in atm?
Given: R = 0.0821 L. atm/mol. K
O 9.8 atm
O 0.00069 atm
O 0.82 atm
O 0.0082 atm

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
A sample of 2.0 moles of helium gas is contained in a tank with a volume of 5.0L at a temperature of...
Questions






English, 26.06.2019 20:20


Mathematics, 26.06.2019 20:20




Mathematics, 26.06.2019 20:20


Mathematics, 26.06.2019 20:20

English, 26.06.2019 20:20