
Chemistry, 13.05.2021 17:00 SmoothCruzito16
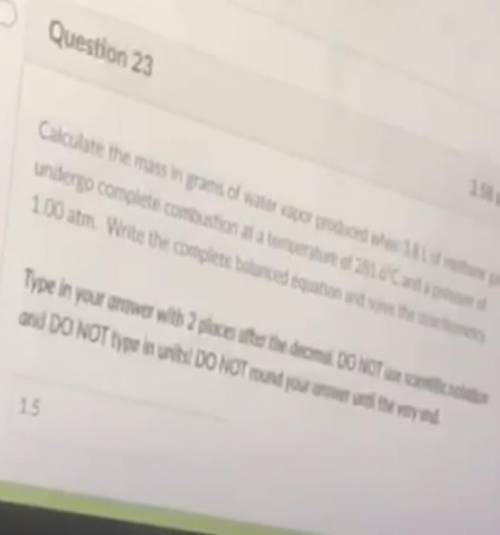
Calculate the mass in grams of water vapor produced when 1.8 L of methane gas undergo complete combustion at a temperature of 281.6°c and a pressure of 1.00 atm. Write the complete balance equation and solve the stoichometry.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1


Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
Calculate the mass in grams of water vapor produced when 1.8 L of methane gas undergo complete combu...
Questions


Mathematics, 11.08.2021 16:10




Mathematics, 11.08.2021 16:10

Mathematics, 11.08.2021 16:10

English, 11.08.2021 16:10

History, 11.08.2021 16:10

Mathematics, 11.08.2021 16:10



Physics, 11.08.2021 16:10



Mathematics, 11.08.2021 16:10


English, 11.08.2021 16:10

Chemistry, 11.08.2021 16:10

Mathematics, 11.08.2021 16:10



