
Chemistry, 13.05.2021 19:10 bethyboop1820
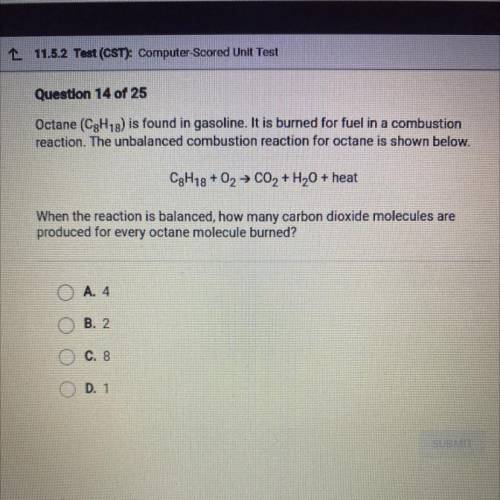
Octane (C8H18) is found in gasoline. It is burned for fuel in a combustion
reaction. The unbalanced combustion reaction for octane is shown below.
C8h18+ O2 → C02 + H2O + heat
When the reaction is balanced, how many carbon dioxide molecules are
produced for every octane molecule burned?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3
You know the right answer?
Octane (C8H18) is found in gasoline. It is burned for fuel in a combustion
reaction. The unbalanced...
Questions


Biology, 20.07.2019 05:40


Biology, 20.07.2019 05:40


Biology, 20.07.2019 05:40

Biology, 20.07.2019 05:40

Biology, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Biology, 20.07.2019 05:40



Social Studies, 20.07.2019 05:40

Computers and Technology, 20.07.2019 05:40

Biology, 20.07.2019 05:40

Social Studies, 20.07.2019 05:40

Business, 20.07.2019 05:40

History, 20.07.2019 05:40

History, 20.07.2019 05:40



