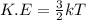Chemistry, 13.05.2021 20:00 osuigwewhitney487
PLEASE HELP
The picture shows two containers filled with a gas, both initially at room temperature.
Which statement is correct? (5 points)
1) The average kinetic energy of the gas particles is greater in container A because its particles move faster.
2)The average kinetic energy of the gas particles is greater in container Bbecause it has a lower temperature.
3) The gas particles in both containers have the same average kinetic energy
because they have the same volume.
4)The gas particles in both containers have the same average kinetic energy
because they have equal number of particles.


Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1
You know the right answer?
PLEASE HELP
The picture shows two containers filled with a gas, both initially at room temperature....
Questions



Mathematics, 23.10.2021 06:00



History, 23.10.2021 06:00

Computers and Technology, 23.10.2021 06:00


Mathematics, 23.10.2021 06:00


Mathematics, 23.10.2021 06:00



History, 23.10.2021 06:00



Mathematics, 23.10.2021 06:00