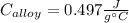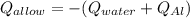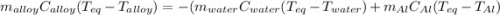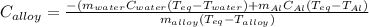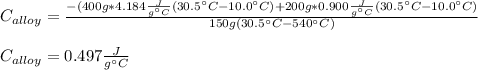Chemistry, 13.05.2021 20:40 ptanner706
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water at 10.0 Celsius, which is contained in a 0.200-kg aluminum calorimeter cup. The final temperature of the system is 30.5 Celsius. What is the specific heat of the metal alloy in J/Kg. Celsius

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the percentage by mass of silicon (si) in iron aluminum silicate (fe3al2(sio4)3)?
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water...
Questions

Advanced Placement (AP), 10.03.2021 15:40



English, 10.03.2021 15:40

History, 10.03.2021 15:40



English, 10.03.2021 15:40




Mathematics, 10.03.2021 15:40

Chemistry, 10.03.2021 15:40

Mathematics, 10.03.2021 15:40


Arts, 10.03.2021 15:40



Chemistry, 10.03.2021 15:40