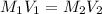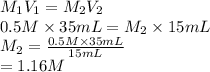Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 22.06.2019 23:30
Match each statement with the state of matter it describes
Answers: 3
You know the right answer?
If 35 mL of a .5 m hydrochloric acid solution is required to neutralize 15 mL of a sodium hydroxide...
Questions

English, 31.10.2020 02:50

English, 31.10.2020 02:50


Geography, 31.10.2020 02:50

History, 31.10.2020 02:50


Physics, 31.10.2020 02:50




Mathematics, 31.10.2020 02:50

Social Studies, 31.10.2020 02:50


Mathematics, 31.10.2020 02:50

Mathematics, 31.10.2020 02:50



Mathematics, 31.10.2020 02:50

Chemistry, 31.10.2020 02:50

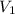 = 35 mL,
= 35 mL, 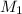 = 0.5 M
= 0.5 M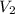 = 15 mL,
= 15 mL, 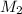 = ?
= ?