
Chemistry, 13.05.2021 22:20 angieplasencia8
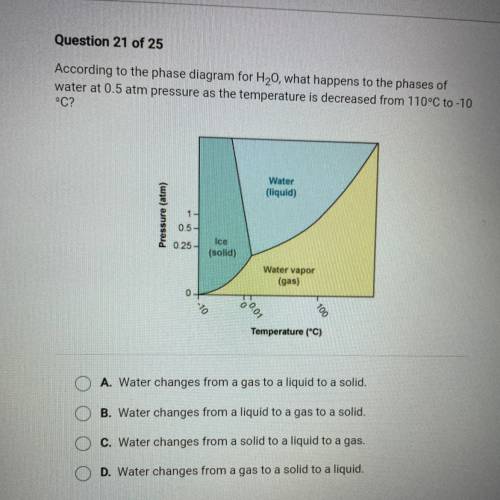
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as the temperature is decreased from 110°C to -10
°C?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as...
Questions


Biology, 08.12.2020 17:30

Mathematics, 08.12.2020 17:30

Mathematics, 08.12.2020 17:30



Arts, 08.12.2020 17:30

Engineering, 08.12.2020 17:30

English, 08.12.2020 17:30

Mathematics, 08.12.2020 17:30

Physics, 08.12.2020 17:30

Computers and Technology, 08.12.2020 17:30


Mathematics, 08.12.2020 17:30



Biology, 08.12.2020 17:30

Mathematics, 08.12.2020 17:30

Social Studies, 08.12.2020 17:30



