
Chemistry, 14.05.2021 02:10 brooklynunderwood46
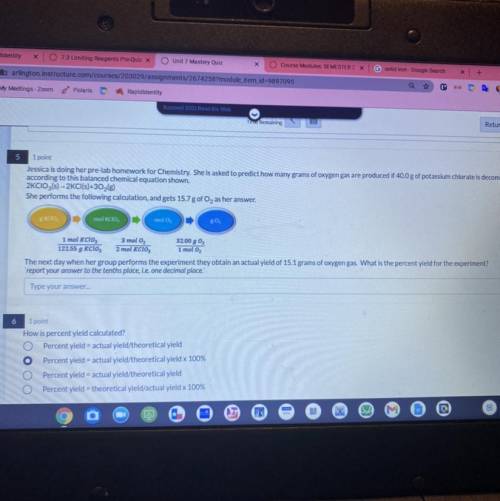
Jessica is doing her pre-lab homework for Chemistry. She is asked to predict how many grams of oxygen gas are produced if 40.0 g of potassium chlorate is decomposed
according to this balanced chemical equation shown.
2KCIO3(s) - 2KCI(s)+302(g)
She performs the following calculation, and gets 15.7 g of O, as her answer.
EKCIO, ,
mol KCIO,
molo
80
1 mol Kclo 3 mol O2
32.00 g 02
122.55 g KCIO, 2 mol KCIO, 1 mol O2
The next day when her group performs the experiment they obtain an actual yield of 15.1 grams of oxygen gas. What is the percent yield for the experiment?
report your answer to the tenths place, i. e.one decimal place.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2


Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1
You know the right answer?
Jessica is doing her pre-lab homework for Chemistry. She is asked to predict how many grams of oxyge...
Questions

Mathematics, 20.03.2020 00:42

Mathematics, 20.03.2020 00:43



Mathematics, 20.03.2020 00:43

Mathematics, 20.03.2020 00:43




Mathematics, 20.03.2020 00:43

Mathematics, 20.03.2020 00:43

Mathematics, 20.03.2020 00:44

English, 20.03.2020 00:44



Computers and Technology, 20.03.2020 00:44



Mathematics, 20.03.2020 00:44




