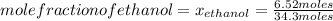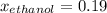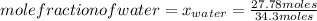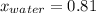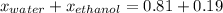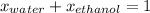Chemistry, 14.05.2021 03:10 joseperez1224
What is the mole fraction of each component in a solution made by mixing 300 g of ethanol(C2H5OH) and 500 g of water?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
What is the mole fraction of each component in a solution made by mixing 300 g of ethanol(C2H5OH) an...
Questions


Computers and Technology, 20.03.2020 23:40

Mathematics, 20.03.2020 23:40


English, 20.03.2020 23:40

Mathematics, 20.03.2020 23:41

Mathematics, 20.03.2020 23:41




Physics, 20.03.2020 23:42




English, 20.03.2020 23:42


Social Studies, 20.03.2020 23:42

Physics, 20.03.2020 23:42

Computers and Technology, 20.03.2020 23:42

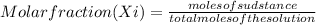
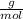 Water 18
Water 18 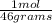 = 6.52 moles
Moles of water: 500 grams*
= 6.52 moles
Moles of water: 500 grams* 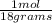 = 27.78 moles
= 27.78 moles