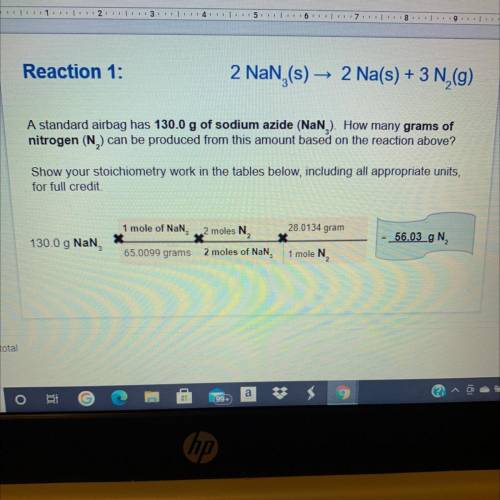
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of so...

Chemistry, 14.05.2021 05:40 lescoto9035
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of sodium produced from reaction 1, how many of grams of
nitrogen will also be produced in reaction 2?
+
Show your stoichiometry work in the tables below, including all appropriate units,
for full credit
gN
46 g Na
This slide is worth 2.5 pts total

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Questions



Mathematics, 09.11.2020 05:10

English, 09.11.2020 05:10

Mathematics, 09.11.2020 05:10


Mathematics, 09.11.2020 05:10


Mathematics, 09.11.2020 05:10

History, 09.11.2020 05:10



English, 09.11.2020 05:10

Biology, 09.11.2020 05:10



Biology, 09.11.2020 05:10





