

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
2 C6H14 + 19 O2 --> 12 CO2 + 14 H2O
Given 250 g of Oxygen and 50 grams of CoH14 what is the maxi...
Questions


Mathematics, 01.02.2021 18:00


Mathematics, 01.02.2021 18:00


Geography, 01.02.2021 18:00

English, 01.02.2021 18:00

Mathematics, 01.02.2021 18:00

Mathematics, 01.02.2021 18:00



History, 01.02.2021 18:00


Mathematics, 01.02.2021 18:00



Mathematics, 01.02.2021 18:00



Health, 01.02.2021 18:00

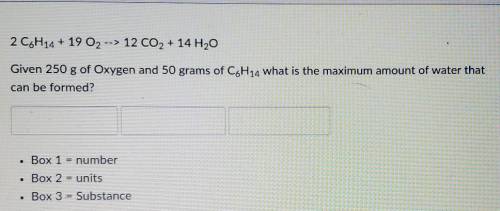
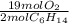 = 5.51 mol O₂
= 5.51 mol O₂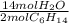 = 4.06 mol H₂O
= 4.06 mol H₂O

