ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
...

Chemistry, 14.05.2021 18:00 shartman22
ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
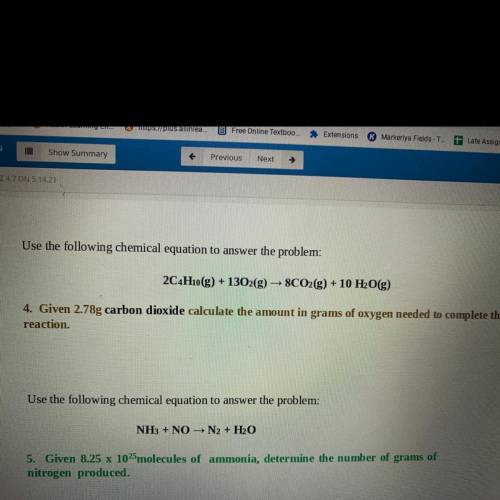
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g)
4. Given 2.78g carbon dioxide calculate the amount in grams of oxygen needed to complete the
reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 08:30
This has nothing to do with school. i wrote a poem to my crush, who i'm asking out soon. tell me if it's cheesy, or cute. "roses are red, violets are blue no love story sounds right if it doesn't include you. dance with me all night, gaze into my eyes i'll hand you my heart, as well as my pride. when i hear your name, my heart goes insane. your all that i want, all that i need promise me you'll stay with me. here it is the final line, jasmine hill will you be mine? " i'm also going to buy her flowers, teddy bear and some food lol. written by me, bre (:
Answers: 2
You know the right answer?
Questions


Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32



Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32


Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32


Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32

Mathematics, 01.04.2020 04:32

English, 01.04.2020 04:32


English, 01.04.2020 04:32



