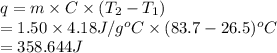Chemistry, 14.05.2021 18:40 shanicar33500
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required to heat 1.50 g of water from 26.5oC to 83.7oC. (Ignore significant figures for this problem.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required...
Questions


Biology, 07.12.2021 01:00




Mathematics, 07.12.2021 01:00


Mathematics, 07.12.2021 01:00

Chemistry, 07.12.2021 01:00


Social Studies, 07.12.2021 01:00

Mathematics, 07.12.2021 01:00


Engineering, 07.12.2021 01:00

Chemistry, 07.12.2021 01:00


SAT, 07.12.2021 01:00


Mathematics, 07.12.2021 01:00

Business, 07.12.2021 01:00

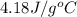
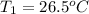
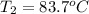
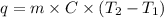
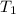 = initial temperature
= initial temperature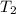 = final temperature
= final temperature