
Chemistry, 14.05.2021 20:10 jazzycintron14
Geologists can estimate the age of rocks by their uranium-238 content. The uranium is incorporated in the rock as it hardens and then decays with first-order kinetics and a half-life of 4.5 billion years. A rock is found to contain 83.9% of the amount of uranium-238 that it contained when it was formed. (The amount that the rock contained when it was formed can be deduced from the presence of the decay products of U-238.) How old is the Rock?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1



Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
Geologists can estimate the age of rocks by their uranium-238 content. The uranium is incorporated i...
Questions


Advanced Placement (AP), 12.04.2021 20:20

Mathematics, 12.04.2021 20:20

English, 12.04.2021 20:20



Mathematics, 12.04.2021 20:30

Mathematics, 12.04.2021 20:30

English, 12.04.2021 20:30


Chemistry, 12.04.2021 20:30

Chemistry, 12.04.2021 20:30




Mathematics, 12.04.2021 20:30

Mathematics, 12.04.2021 20:30

Mathematics, 12.04.2021 20:30

English, 12.04.2021 20:30

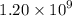 years
years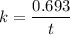
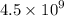 years
years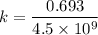
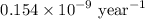
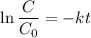
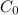 = initial amount
= initial amount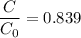
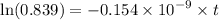
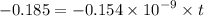
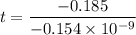
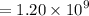 years
years


