
Chemistry, 14.05.2021 21:50 Kittylover4812
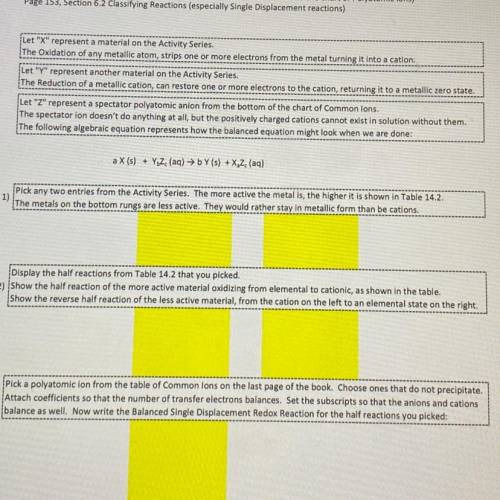
Let "X" represent a material on the Activity Series
The Oxidation of any metallic atom, strips one or more electrons from the metal turning it into a cation.
Let "Y" represent another material on the Activity Series.
The Reduction of a metallic cation, can restore one or more electrons to the cation, returning it to a metallic zero state.
Let "Z" represent a spectator polyatomic anion from the bottom of the chart of Common Ions.
The spectator ion doesn't do anything at all, but the positively charged cations cannot exist in solution without them.
The following algebraic equation represents how the balanced equation might look when we are done:
a X (s) + YZ (aq) → bY (s) + X. Z (aq)
1)
Pick any two entries from the Activity Series. The more active the metal is, the higher it is shown in Table 14.2.
The metals on the bottom rungs are less active. They would rather stay in metallic form than be cations
Display the half reactions from Table 14.2 that you picked
2) show the half reaction of the more active material oxidizing from elemental to cationic, as shown in the table.
show the reverse half reaction of the less active material, from the cation on the left to an elemental state on the right.
Pick a polyatomic ion from the table of Common ions on the last page of the book. Choose ones that do not precipitate.
3) Attach coefficients so that the number of transfer electrons balances. Set the subscripts so that the anions and cations
balance as well. Now write the Balanced Single Displacement Redox Reaction for the half reactions you picked:

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
Let "X" represent a material on the Activity Series
The Oxidation of any metallic atom, strips one...
Questions

Mathematics, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Arts, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50

Physics, 17.11.2020 19:50




Mathematics, 17.11.2020 19:50




English, 17.11.2020 19:50



Mathematics, 17.11.2020 19:50


Mathematics, 17.11.2020 19:50


Biology, 17.11.2020 19:50



