Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3
You know the right answer?
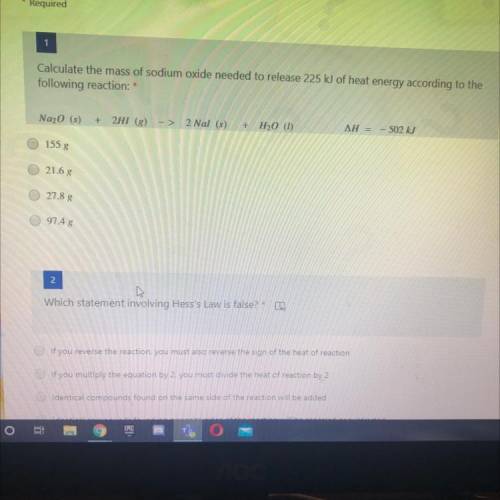
Calculate the mass of sodium oxide needed to release 225 kJ of heat energy according to the
followi...
Questions

Mathematics, 21.04.2021 15:40

World Languages, 21.04.2021 15:40

Mathematics, 21.04.2021 15:40

Chemistry, 21.04.2021 15:40



Mathematics, 21.04.2021 15:40

Mathematics, 21.04.2021 15:40


Mathematics, 21.04.2021 15:40


Mathematics, 21.04.2021 15:40






Mathematics, 21.04.2021 15:40