
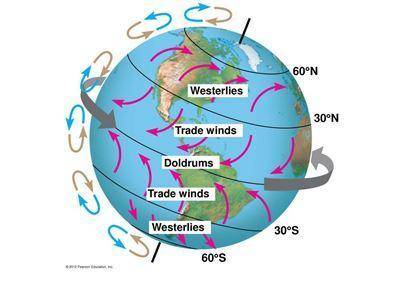
Students in a science class were asked to develop a model to show how global winds form on Earth. Many students drew the same model above but did not correctly explain how global winds form. Which student has developed the correct explanation for the model to show how global winds form on Earth
A.
Student 2: Low pressure replaces high pressure causing global wind to form and the spin of the Earth causes these winds to curve.
B.
Student 3: High pressure and low pressure become equal causing global wind to form and the spin of the Earth causes these winds to curve.
C.
Student 4: High pressure and low pressure mix together causing global wind to form and the spin of the Earth causes these winds to curve.
D.
Student 1: High pressure replaces low pressure causing global wind to form and the spin of the Earth causes these winds to curve.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1
You know the right answer?
Students in a science class were asked to develop a model to show how global winds form on Earth. Ma...
Questions



Mathematics, 17.12.2019 18:31
















Mathematics, 17.12.2019 18:31




