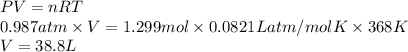Chemistry, 15.05.2021 02:00 leomessifanboy678
Hydrogen sulfide gas can be produced from the reaction of aluminum sulfide and water according to the following equation: Al2S3(s) 6H2O(l) > 3 H2S(g) 2Al(OH)3(aq) What volume of hydrogen sulfide gas is produced if 65.0 g Al2S3 reacts at 90.0 oC and 0.987 atm,

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
Hydrogen sulfide gas can be produced from the reaction of aluminum sulfide and water according to th...
Questions



Mathematics, 01.10.2019 08:30


Mathematics, 01.10.2019 08:30

Chemistry, 01.10.2019 08:30




Mathematics, 01.10.2019 08:30


History, 01.10.2019 08:30

History, 01.10.2019 08:30

Mathematics, 01.10.2019 08:30

Mathematics, 01.10.2019 08:30

Mathematics, 01.10.2019 08:30

Health, 01.10.2019 08:30


Mathematics, 01.10.2019 08:30

Mathematics, 01.10.2019 08:30

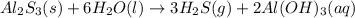
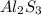 is given as 65.0 g. Hence, moles of
is given as 65.0 g. Hence, moles of 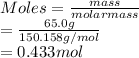
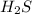 . Hence, moles of
. Hence, moles of 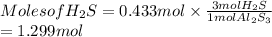
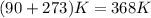 . Therefore, volume of hydrogen sulfide is calculated as follows.
. Therefore, volume of hydrogen sulfide is calculated as follows.