
Chemistry, 17.05.2021 01:50 timothycarter342
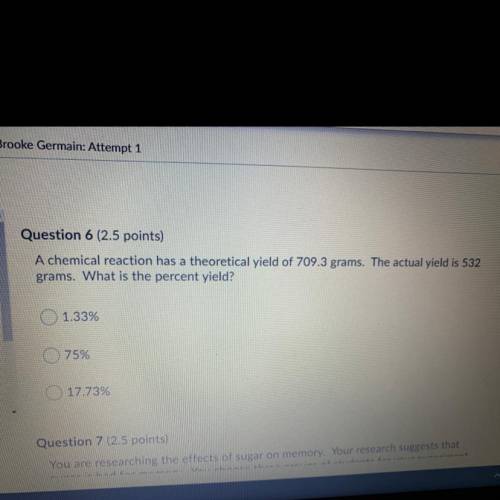
A chemical reaction has theoretical yield of 709.3 grams. The actual yield is 532 grams. What is the percent yield?
1.33%
75%
17.73%

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
A chemical reaction has theoretical yield of 709.3 grams. The actual yield is 532 grams. What is the...
Questions

Physics, 04.02.2020 01:48

English, 04.02.2020 01:48


Mathematics, 04.02.2020 01:48

Mathematics, 04.02.2020 01:48

Mathematics, 04.02.2020 01:48


Mathematics, 04.02.2020 01:48



Mathematics, 04.02.2020 01:48

Chemistry, 04.02.2020 01:48



Business, 04.02.2020 01:48




English, 04.02.2020 01:48



