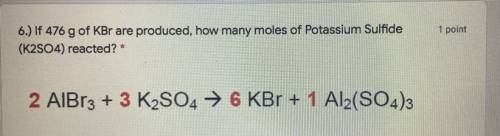
6.) If 476 g of KBr are produced, how many moles of Potassium Sulfide
(K2SO4) reacted? *
2 Al...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
Questions


Mathematics, 20.04.2020 02:41

Physics, 20.04.2020 02:41



Chemistry, 20.04.2020 02:41

Mathematics, 20.04.2020 02:41

Mathematics, 20.04.2020 02:41

Mathematics, 20.04.2020 02:41



Chemistry, 20.04.2020 02:41


Mathematics, 20.04.2020 02:41

Mathematics, 20.04.2020 02:41

Mathematics, 20.04.2020 02:42

Mathematics, 20.04.2020 02:42


Biology, 20.04.2020 02:42