
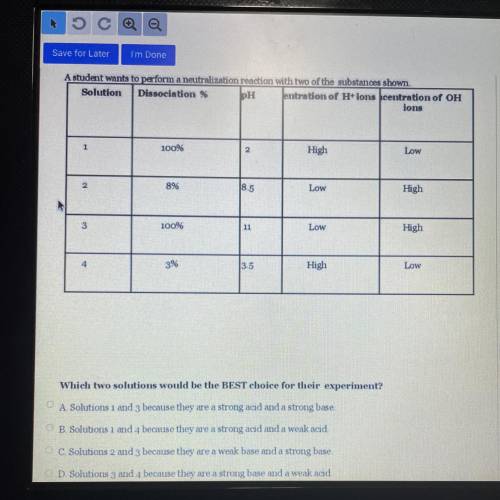
A student wants to perform a neutralization reaction with two of the substances shown.
Solution Dissociation % PH Jentration of H+ ions centration of OH
ions
100%
2
High
Low
8%
8.5
Low
High
3
100%
11
Low
High
4
3%
13.5
High
Low
Which two solutions would be the BEST choice for their experiment?
A Solutions 1 and 3 because they are a strong acid and a strong base
B. Solutions 1 and 4 because they are a strong acid and a weak acid
C. Solutions 2 and 3 because they are a weak base and a strong base
D Solutions 3 and 4 because they are a strong base and a weak acıd.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1


Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1
You know the right answer?
A student wants to perform a neutralization reaction with two of the substances shown.
Solution Dis...
Questions


Chemistry, 18.07.2019 19:00

Social Studies, 18.07.2019 19:00




Physics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00


Mathematics, 18.07.2019 19:00

Spanish, 18.07.2019 19:00

English, 18.07.2019 19:00

History, 18.07.2019 19:00


Mathematics, 18.07.2019 19:00

Mathematics, 18.07.2019 19:00


History, 18.07.2019 19:00

History, 18.07.2019 19:00



