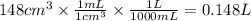Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
3. At 34.0°C, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm3 is 212
kPa...
Questions

Computers and Technology, 07.11.2020 05:40

World Languages, 07.11.2020 05:40





Mathematics, 07.11.2020 05:40


Mathematics, 07.11.2020 05:40




Mathematics, 07.11.2020 05:40


Chemistry, 07.11.2020 05:40


English, 07.11.2020 05:40